Just a bit of fun really; but I’ve been mugging up on the basics of CGI. Things sure have moved on from the days of Muybridge and flip books ! Here’s my first attempt:
Movie made in DAZ Studio 4.6 with Animate2 plug-in.
Just a bit of fun really; but I’ve been mugging up on the basics of CGI. Things sure have moved on from the days of Muybridge and flip books ! Here’s my first attempt:
Movie made in DAZ Studio 4.6 with Animate2 plug-in.
It’s still the holiday season, so no apologies for doodling on about gingerbread, which, as it turns out, can be pretty strong stuff – if a bit bendy.
Cue my wife Erin’s first attempt at a gingerbread house (above). Pretty good, huh? The heat from the incandescent fairy lights has kept it from turning mushy, and nicely spiced up the room at the same time. The house is only eight inches tall, but prompts the obvious question: “How high can you build with gingerbread?”
A structural analysis of a full-on house with walls, windows and doors is too tall an order, even with finite element techniques, so I settled on calculating a ballpark maximum height based on standard engineering equations for a free-standing gingerbread column.
There’s no wind blowing through our lounge, so we can ignore sideways forces and focus on the two likely failure modes a column of gingerbread might suffer – just because of its own weight as it gets taller, i.e.:
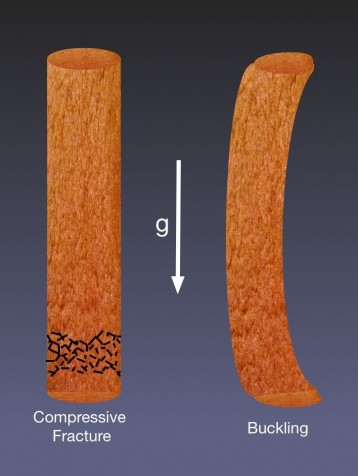 (a) the construction materials can disintegrate under their own weight: a function of compressive strength, or
(a) the construction materials can disintegrate under their own weight: a function of compressive strength, or
(b) the column can buckle, which is more related to the material’s elastic, or tensile properties.
The heights at which these two failures occur can be found from, respectively:
where =column height at compressive failure (m),
is the failure pressure (N/m2) = compressive strength of the gingerbread, g=gravity (9.8 ms-2), and
is gingerbread density (kg m-3). And for buckling:
is the critical height, E is Young’s Modulus of elasticity calculated as tensile stress/strain, I is the Area Moment of Inertia3, and
is a factor called a Bessel function, used to solve this type of equation (Ref.2)
Using published gingerbread properties data1 (amazingly, there actually are some) for compressive strength and tensile stress/strain, I calculated values of:
(Workings in box below if you’re interested.)
which essentially means a gingerbread column will start to lean over and buckle sideways long before the gingerbread breaks up through compression under its own weight (I used an arbitrary but realistic 20 cm column diameter). You might think there’s no reason why a uniform, vertical, column would start to lean, but in real life the weight distribution is never uniform and, if the column is sufficiently slim, a turning moment will establish and drive a progressive buckle.
So if you’re going to build a gingerbread house out of free-standing columns, better stop at 3 metres.
Buckling is clearly the limiting factor, but the 3 metre figure is based on a relatively small 0.2m column diameter, and buckling is particularly sensitive to cross-sectional area (whereas compressive fracture of a column under its own weight is independent of area). Also, most real buildings are more complex than a bunch of pillars, and I’d expect the right combination of interconnecting members building up from a broad foundation could reduce buckling potential, making a full-size gingerbread house a reality.

Indeed, the Guinness Book of Records ‘Worlds largest gingerbread house’ is 18.28m (60ft) on a 13.86m by 10.8m base; but closer inspection shows it’s built around a steel frame that presumably keeps incipient buckling in check. But then it’s more of a gingerbread and steel house – a bit of a con really.
Anyhow, our room’s about 3 metres high, so nothing stopping a more ambitious project next year: Empire State Building or Cathédrale Notre Dame ?
Workings
Note that for compressive failure of a column under its own weight, the area of the column A (m2) cancels and isn’t relevant: i.e so,
and
as above.
I couldn’t measure my own gingerbread density easily (although it for sure floated in water, so < 1000 kg m-3), and used a middle value of 700 kg m-3 from this unlikely study by students at the University of British Columbia (UBC)1. In addition to the UBC data for of 346 kPa, I measured my own value for
by pressing a sample (squirrel-shaped in this case, but taking the narrowest foot area as 1*10-4m2) vertically downwards onto a balance and recording when it crumbles.
 Still intact when the balance read 6kg, I took my
Still intact when the balance read 6kg, I took my to be at least 6 * 9.8 / 1*10-4, or 588 * 103 N/m2 (588 kpascal kPa). In fact, for compressive strength, my numbers and the published data are conservative, as in neither case did the gingerbread actually fail at these values. So:
(UBC data)
(my gingerbread)
Whether it buckles first, at a lower height, depends on the elasticity of the gingerbread and the slenderness of the column: i.e. the ratio of column area to length.
The height at which buckling occurs can be found from Cox & McCarthy2:
where is the critical height for buckling, E is Young’s Modulus of elasticity calculated as tensile stress/strain, and I is the Area Moment of Inertia3.
To calculate , I chose an arbitrary column diameter of 20 cm diameter, and used stress/strain data from the Canadian study1 to calculate E = 9790 kPa; i.e. 219/0.02237 (the change in dimensions of my squirrel under tension are too small to measure with the kit I have).
The Area Moment of Inertia for a circular cross-section , which for a 0.2m dia. column gives
. And (
, is the appropriate Bessel function of order -1/3 (Ref.2) Note: in the source equation, weight density is specified; hence g added here.) So:
A more complex structure would best be assessed computationally using finite element analysis, but I’m not getting into that.
References
1. ‘Building with gingerbread: Engineering students put holiday delight to the test’ refers to ‘Structural Analysis of Gingerbread. Engineering Design Project Term 2’ by Mercedes Duifhuis and Sean Heisler (pdf)
2. The Shape of the Tallest Column. Steven J.Cox, C.Maeve McCarthy, Society for Industrial and Applied Mathematics. Vol29,No.3. pp.547-554. (Also see Wikipedia page on buckling.)
3. Engineering Fundamentals efunda.com/math)
Of related interest
How to create the perfect sand castle Nature Scientific Reports 2, Article number:549, doi:10.1038/srep00549
You may have seen swans performing various synchronised movements or ‘dances’ together on the water at mating time.
The pre-copulatory rituals extend to preening, and although we’re out of the breeding season, this local pair I snapped this afternoon are clearly keeping in practice. Their synchronised stink-eye, reserved for loose dogs and over-eager photographers, is pretty impressive too.




Maybe it was the furnace heat of California last month, or the topicality of NASA’s Curiosity landing, but here I am having my first – and almost certainly last – von Däniken moment.


How else though, aside from some ancient Martian visitation, could Native Americans of centuries past, without the benefit of telescopes or interplanetary probes, design water pots so closely matching the Red Planet?
Well, on reflection, I guess a mixture of clay and cactus juice might just bake out that way in the sun.
Which brings us to the real science behind these earthenware pots. Because although they may well be over two hundred years old, discovered in 1926 by my wife’s geologist great-grandfather in the desert of San Diego County, these water carrying olla represent nothing less than the world’s first refrigerator.

The water inside the olla reaches a temperature substantially below that of the surroundings thanks to the principle of evaporative cooling – something you can demonstrate to yourself just by licking a finger and waving it around. The skin feels cooler because the heat needed to turn liquid water molecules in your spit into vapourised water molecules leaving your hand is taken from your skin. The amount of heat, or energy, needed to change from a liquid to a gas is called the latent heat of evaporation, which for water is 2257 kJoules per kilogram.

The sun-baked porous clay of the olla acts like a wick, delivering a constant flow of evaporating water to the surface where it quickly evaporates, cooling first the surface and in turn the water inside the pot.
Wondering how effective ollas really are, but with live tests on our delicate pots off the agenda, I turned to theoretical musings and some (not entirely successful) experimentation.
The Theory
The temperature on the pot’s surface, or wet-bulb temperature, is easy enough to calculate if we know the ambient air temperature, relative humidity (how much water is already in it), and local air pressure – as that affects the dew-point temperature at which water changes from liquid to gas. I got all that info from my local weather station online, and plugged it into one of the many online calculators – like this one at the National Oceanic and Atmospheric Administration (NOAA) – to find the wet bulb temperature. (The exact calculation is complex and explained on the NOAA website, but essentially the drier the air, the lower the wet-bulb temperature; water molecules already in the air decrease the net evaporation rate.)
The day I looked at this, the values were: temperature 34 C, relative humidity 20%, and air pressure 1014.9 millibars, for which the NOAA calculator returned a wet-bulb temperature of about 19 C. That’s a whole 15 degrees below ambient temperature; modern electric fridges don’t do much better than that (okay – granted they can get to lower absolute temperatures).

The wet-bulb temperature I verified experimentally using a cooking thermometer modified with wet paper-towel stuffed in around the sensor tip (a mercury thermometer would have a bulb of mercury at the end – hence wet-bulb; but this was all I had and works well enough). Swinging the thing fast round my head on the end of a shoelace simulated wind and, lo and behold, I recorded a wet-bulb temperature of 21C. Not quite the predicted 19C, but in the right area.
Good ventilation of the olla is necessary as it influences the evaporation rate, and the area for evaporation should be large (the olla’s spherical design is fantastic in this regard as it maximises the area). The area of non-wetted contact should be small to minimise absorption of heat by conduction from the surroundings – here again, the point contact of the spherical olla is perfect. Ollas also work better in the shade, to minimise heating by solar radiation.
Calculating how long the contents take to cool is more tricky, requiring an estimate of the evaporation rate from a porous surface. But we can get some handle on it using an assumed rate of 7kg/m2.day (based on some data I found for swimming pool evaporation rates in Australia of all things), latent heat of evaporation of water 2257 kJ/kg, and heat capacity of water 4.18 kJ/kg.K. From which I reckon the 0.3m diameter olla, holding 14 litres (=14 kg) of water, needs to lose 878 kJ of heat to fall in temperature by 15 degrees, equal to evaporating 0.4 litres (0.4kg or 3% of the contents) from the 0.28 m2 surface over a 5 hour period.
The numbers aren’t perfect, but suggest in its heyday our olla was up and usefully cooling in a couple of hours.
The Practise
Now for the not-totally-successful experimentation part of the post.

You can see what I’m trying to do here: my very own plant pot olla. The physical conditions (temp.,humidity,pressure) were the same as the theoretical calculation; and I’d confirmed a wet-bulb temperature of 22C as described above. The pot was kind of working too; that dark band in the middle and top is water seeping through the porous terracotta – and it was pretty consistent throughout the experiment.
Stirring the contents and taking regular measurements indicated a one degree fall over the first two hours. But then the temperature started to climb again, which suggests the pot was just not porous enough over sufficient area to counter heating by conduction through the non-wetted areas. A lack of wind won’t have helped – maybe use a fan next time. At least no one can accuse me of selectively publishing only positive results.
Other Coolers
The Arab Zeer works in a similar fashion to the olla, but consists of two pots separated by wet sand. Fruit and other perishables can be kept fresh in the central pot.

A modern invention is this Terracooler: an evaporatively cooled terracotta bell-jar placed over food to keep it fresh.

And keep an eye out for artificial waterfalls used to create a cool atmosphere in public spaces, or the same principle operating in simple garden water features: the water in this one I measured at 24C on a 32C day.
I’m back in the UK now, where typical humidity levels close to 100% (=rain) preclude the extensive program of further evaporative cooling tests this discussion clearly signposts. If you have more luck with your own ollas though, do let me know.
You never know what unexpected quirky stuff is going to show up if you keep your eyes open.

This afternoon, Erin and I visited the Pasadena Museum of California Art to see an exhibition of works by Edgar Payne. We’re both fans of American plein-air painting, and Payne was a master of the technique – so the exhibition was a great success. But parking up, we found the Museum’s garage had its own artistic charm.
The graffiti is by artist Kenny Scharf, and instantly caught my eye with its images of rocket ships and swirling galaxies. The garage – or Kosmic Kavern – is the colorful legacy of an exhibition of Scharf’s work in the gallery proper in 2004 – his graffiti in the garage was just never cleaned off! Scharf’s work is influenced by the 1962 animated comedy sit-com The Jetsons, and there are other bits of space and nuclear iconography from the Golden Age of American Science spotted around – like the mushroom cloud and atom-swirl.


Some of the Jetson’s techno-utopia became a reality. But not, unfortunately, the aerocar or three-day week.
More Kenny Scharf
If you’d like to see more of his Kenny Scharf’s work, there’s a good collection at Artsy’s Kenny Scharf Page
Of related interest on Zoonomian
It’s that candlelit dinner stage of the evening. Soup through nuts, you’ve been your wonderful, genuine, self. And he/she is pretty fantastic too.

But why take chances – this is deal clinching time.
With the table cleared, quick as a whippet you pull out your Ethereal Collapse CD, and with a flourish Newton would die for if he wasn’t already dead, guide your beloved’s eye to the spectacular demonstration of spectra by diffraction.
Your friend will by now be in a frenzy of excitement, so this is the moment to push them over the edge.
Rushing through to the restaurant kitchen with a mix of urgency and discord normally reserved for Bond movies, you thrust your CD into the light once again. But now the disc reflects the chef’s fluorescent tube in an almost unbearably different, and extremely interesting way. The smooth continuum of the candle flame is gone! Now superposition bands stand proud, where line discharge spectra from gaseous mercury inside the lamp combine with the continuous spectra emitted from the phosphor coating.

At this point, you’ll almost certainly be offered complimentary Cognacs – if only to leave the kitchen. But by now you’ll both be itching to get off anyway, back to his/her flat to repeat the experiments under controlled conditions. Or maybe play some Scrabble.
Note: Humphry Davy was up for colour ploys (Link to ‘Humphry Davy – Finding Love in the Colourful Age of Romantic Science’)
Those crazy Victorian inventors. What can you do with them? Whenever I research a history project, some totally unconnected but wonderful distraction like this shows up and wants sharing.
Maybe that’s how inventor James Wilcox thought about his ‘profile likeness’ doorknob keepsake idea from the Victorian era, reported in an 1838 edition of (take a deep breath) the Mechanics’ Magazine, Museum, Register, Journal and Gazette: one of the many popular journals at the time disseminating a veritable gush of 19th century science and engineering.
At first glance I thought this was about profiling a door knob and sticking it back on the door; but that wouldn’t really work – would it. No, Wilcox’s breakthrough technology involves filing the profile of your head into a steel tool, and using it to turn a likeness into an old wooden doorknob. Cutting the thus-profiled piece into slices gives you a whole bunch of keepsakes for handing out to friends and acquaintances. Inspired!
Okay, I’m taking the Mickey a bit because it’s such an off-the-wall thing, not to say off-the-door thing, to spend your time on. But in all seriousness, there seem to have been a lot of men (at least mainly men were visible) like Wilcox around, who, variously tucked away in workshops, cottages, and garden sheds, had the will and wherewithal to have a go at the various engineering challenges of the day. And even if some ideas were silly and others came to nought, ordinary folk still felt they had the right and ability to contribute – although with increasing complexity and specialisation that was becoming ever harder; it’s almost impossible today.
Hey, I like Wilcox’s idea – a kind of wooden business card or Carte de Visite as the Victorians would have it. It’s not like you’d forget a guy who slipped you a slice of his door knob.
I also like Wilcox’s self-effacing humility, where after he says “If this has ever been done before, by any other person, I am not aware of it” he goes on to concede there’s still room for improvement – if only that messy screwed piece at the back was done away with. Ah, the compromises one has to make for rapid prototyping, or as Wilcox explains in value-added detail right to the end “My mandrill having a female screw, I am obliged to screw the piece into it; but with a male screw, the operation can be done much neater.”
There you go. A little whiff of the Great in Great Britain :-P.
Like any normal person, I decorate my bathroom with Victorian engravings of anthropomorphised monkeys.

These two depict the kind of half ape / half-monkey used by Benjamin Brooke and Lever Brothers to promote Monkey Brand soap, a super-popular cleaning product at the turn of the twentieth century.
An intriguing strap line at the bottom of these simian vignettes catches the eye, and, in those contemplative loo moments, piques an interest for further research. It’s a fleeting urge, seconds later flushed from thought – the cycle repeating with each visit. That’s how I know Monkey Brand “WON’T WASH CLOTHES“.

Spotting these blocks of the real thing at the Museum of London prompted further research.

Of more interest than the technical side of Monkey Brand is the way social and cultural historians, public relations types, and advertising scholars have tried to understand the what, why, and wherefore of a marketing strategy underpinned by these pseudo-human creatures.

The gist of these socio-cultural analyses is that the monkey was used (consciously and unconsciously) as symbolic commentary on issues around race, gender and class: representing an idea of change in the Victorian mind that went beyond the obvious clean-dirty associations.
The tens of different Monkey Brand ads produced at this time are a semiotician’s dream. For more on this aspect, check out this post at the ‘Notes on the arts and visual culture blog’.

From the scientific perspective, Monkey Brand ‘Won’t Wash Clothes’ because it was loaded with an abrasive mineral – probably pumice (volcanic ash) – which would almost certainly put a hole through your favourite bib and tucker. Pumice contains shock-cooled glassy particles, making the soap abrasive.
While mostly used on dirty grates and rusty bicycles, there’s a hint of an endorsement for use as an occasional toothpaste, although this story from 1908, of an old lady wearing a hole through her denture plate, suggests that’s not such a good idea.
You can still get something along the lines of Monkey Brand today, this LAVA soap; ;with ‘Pumice Power’: “The hand cleaner of choice for do-it-yourselfers, coal miners, and oil rig workers“.
 Nice to see as early as 1837 Charles Dickens doing his bit to comically debunk the efficacy of infinitesimal doses in medicine. Okay, he’s not quite talking about the bottles of total nothingness you can buy at the chemist and are an insult to reason today, but still interesting that similar ideas were an issue 175 years ago.
Nice to see as early as 1837 Charles Dickens doing his bit to comically debunk the efficacy of infinitesimal doses in medicine. Okay, he’s not quite talking about the bottles of total nothingness you can buy at the chemist and are an insult to reason today, but still interesting that similar ideas were an issue 175 years ago.
This passage from ‘The Mudfrog Pages‘, in the monthly magazine Bentley’s Miscellany, is a fictitious report from the first meeting of the ‘Mudfrog Society for the Advancement of Everything’, a satirical fun-poke at the recently founded British Association for the Advancement of Science :
” Professor Muff related a very extraordinary and convincing proof of the wonderful efficacy of the system of infinitesimal doses, which the section were doubtless aware was based upon the theory that the very minutest amount of any given drug, properly dispersed through the human frame, would be productive of precisely the same result as a very large dose administered in the usual manner. Thus, the fortieth part of a grain of calomel was supposed to be equal to a five-grain calomel pill, and so on in proportion throughout the whole range of medicine. He had tried the experiment in a curious manner upon a publican who had been brought into the hospital with a broken head, and was cured upon the infinitesimal system in the incredibly short space of three months. This man was a hard drinker. He (Professor Muff) had dispersed three drops of rum through a bucket of water, and requested the man to drink the whole. What was the result ? Before he had drunk a quart, he was in a state of beastly intoxication; and five other men were made dead drunk with the remainder. ” The President wished to know whether an infinitesimal dose of soda-water would have recovered them ? Professor Muff replied that the twenty-fifth part of a tea- spoonful, properly administered to each patient, would have sobered him immediately. The President remarked that this was a most important discovery, and he hoped the Lord Mayor and Court of Aldermen would patronize it immediately. ” A Member begged to be informed whether it would be possible to administer — say, the twentieth part of a grain of bread and cheese to all grown-up paupers, and the fortieth part to children, with the same satisfying effect as their present allowance. ” Professor Muff was willing to stake his professional reputation on the perfect adequacy of such a quantity of food to the support of human life — in workhouses; the addition of the fifteenth part of a grain of pudding twice a week would render it a high diet.”

References
The Mudfrog Papers http://www.archive.org/stream/mudfogpapersetcn00dickrich/mudfogpapersetcn00dickrich_djvu.txt
“in Sensation we believe external Things exist, in Memory we believe they were, in Imagination we neither do the one nor the other” (Erasmus Darwin quoting poet Richard Gifford back to himself in a letter of 1768.)
Here’s something to try if you haven’t already done it: make a Google Street View tour of all the old homes you’ve ever lived in.
Of course, if you’ve yet to leave the parental home it’s going to be a dull exercise, but if you’ve been around a while and lived in lots of different places, there’s the joy of reminiscing and spotting that the new owners have gotten around to replacing that leaky porch you ignored all those rainy winters.

It took me half an hour to track down the twelve places I’ve lived in, bought, or rented over the years (some in the pic above); although the flat I lived in for four years in Brussels came out as, well, flat. Belgium seems to have been overlooked by Google Street View)
Apart from the idle interest, dredging the past evokes ideas around the concept of time and how we store information and remember things; although if that’s just me, it’s because I’m presently transitioning between two books that touch on the topic: The Information by James Gleick and Jon Turney’s Winton Prize-longlisted The Rough Guide to the Future.
We capture so much nowadays – Gleick: “The information produced and consumed by humankind used to vanish – that was the norm, the default. The sight, the sounds, the songs, the spoken word just melted away.”
Then came the first marks on paper, drawings, writing; then photographs. Gleick again:
“Now expectations have inverted. Everything may be recorded and preserved, at least potentially: every musical performance; every crime in a shop, elevator, or street; every volcano or tsunami on the remotest shore; every card played or piece moved in an online game; every rugby scrum and cricket match.”
Whether it’s Street View, Flickr, or Friends Reunited, there’s a bunch of stuff pushing in on us, persuading us to reconstruct our pasts in a way that was alien even five years ago.
What does it mean? Is it good?
For sure, any ideas we might have had about ‘clean breaks’ and ‘moving on’ get a good muddying. Old friends: material and personal, reappear unbidden – sometimes welcome, othertimes unsettling away from their original context.
In his chapter About Time, Turney says our memories impact our ability to think about the future; afterall, past experience is pretty much all we have to draw on.
The way we build memories, he says, may have adapted specifically to enable the efficient anticipation of new situations, and there is even evidence of a physical link in how we think about past and future events – neurological scans revealing common areas of brain activity.
Our memories “seem to work by storing individual pieces of past experience separately, as part of a complicated, interconnected web …. Our brains then assemble recollections of past episodes by adding together bits of information that seem to be related.”
As it happens, by Turney’s reckoning, I’m probably at the optimum age for projecting possible futures. Meaning, I’m old enough to have collected some experiences, but not so old I’ve forgotten them all. (I love some of the terminology people use for age brackets, particularly the ‘old old’ – meaning over 80. At 49, I’m holding out for ‘young middle-age’.)
I want to wind up the post by sharing some great life-changing revelations resulting from this technology-induced disturbance in my mental time-space continuum and reassessment of ‘self’. But as the most emotionally charged evocations seem to relate to the unfeasible number of lawnmowers I’ve owned over the years, I’ll skip on that and instead leave you with a bit of topical DNA:
“Time is an illusion. Lunchtime doubly so.”
Douglas Adams. Hitch-hikers Guide to the Galaxy
[follow_me]
Continue reading Blast Through Your Past – with Google Street View