Q – What did the grape say when an elephant stepped on him?
A – Nothing. He just let out a little wine.
The first alcohol I ever drank was home brewed. I was twelve when the evil liquor – orange and raison wine – was served up by my refreshingly enlightened policeman uncle of all people. We’d visit the house and find these wort-filled vessels in the bathroom, glug-glug-glugging as bubbles of carbon dioxide chugged through little glass airlocks.
Not that I was swilling the stuff in quantity you understand, but what better introduction to the practical application of biochemistry and chemical engineering. Who knows what influence these little episodes have on later life decisions?
Six years later, as an impoverished student at Birmingham University, I was brewing my own 40 pints of barely drinkable delicious Mild Ale (pronounced ‘m + oiled’ in the local dialect). And while I never got into the brewing habit big time, I still on occasion reach for the demijohn and yeast – like recently, prompted by the promise of summer blackberries and the pungent whiff of Thames-side hops.
It’s obvious booze is an educational resource we ignore at our peril; but to consolidate, consider what’s going on in that murky ochre, as it sits in my hall, infusing the carpets and curtains with its fruity ambience. I hope it’s this:
C12H22O11 + H2O -> C6H12O6 + C6H12O6
Sucrose Water Glucose Fructose
followed by this:
C6H12O6 -> 2C2H5OH + 2CO2
(Glucose/Fructose) Alcohol (Ethanol) Carbon Dioxide

The contents of the bottle are yellow because the blackberries haven’t actually appeared yet, so for now I’m using Chardonnay grape concentrate out of a can. And as that contains fructose from the grapes plus added glucose syrup, and I’m adding sucrose on top of that, both reactions should have kicked off immediately – the whole thing enabled by one of my favourite eukaryotic micro-organisms – Saccharomyces cerevisiae: a wine yeast.
There’s nothing to do now until it ferments out, but I managed to kill 20 minutes using the chemistry and bubble rate data to figure out how things are ticking along. I reckon I’ll produce 511g of alcohol and 488g (273 litres) of CO2, which at the current bubble rate means the fermentation will take 6 days (workings in the end-notes for those interested and assuming I’ve remembered my O-level chem.).
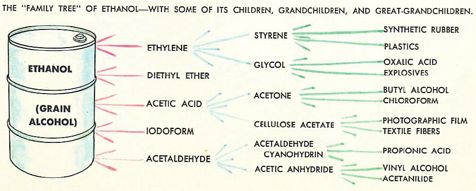
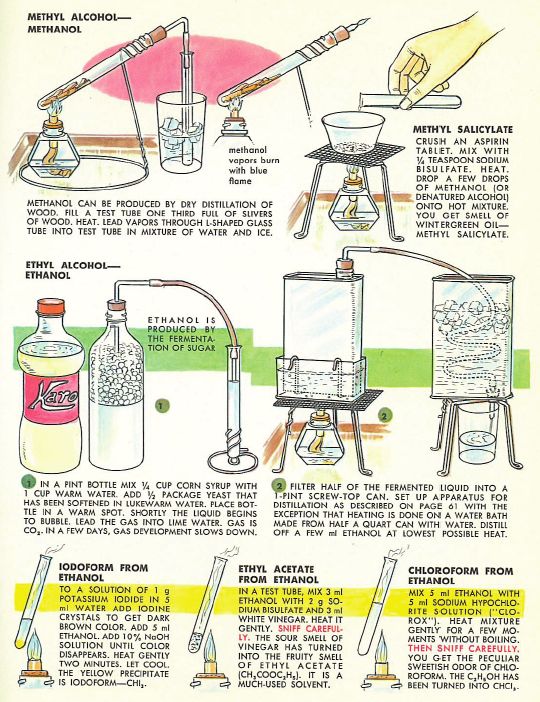
We covered production of ethanol from fermentation at school, but I don’t remember doing any distillation (which is illegal without a license in the UK). Certainly nothing to compare with the alcohol education afforded 1960s American youth courtesy of the fabulous Golden Book of Chemistry Experiments (excerpts below), which covers fermentation with yeast plus the distillation/synthesis of ethanol, methanol, and a bunch of other fun compounds from the ethanol ‘Family Tree’:
I love the helpful precautionary note on chloroform:’THEN SNIFF CAREFULLY’. A complete home schooling if ever there was one:
That’s all really. I’ll update with a report on the finished product, assuming the wrong types of bug and oxygen don’t intervene and vinegarate the show.
One last item though. Yeast is of course also used in baking; the carbon dioxide from fermentation causes dough to rise. So here’s a particularly rigorous explanation of the process from Alton Browne. It’s over my head, but I’m sure the trained biochemists out there will relate. (Quality isn’t up to much either – sorry about that.)
References
The Golden Book of Chemistry Experiments. Robert Brent, Golden Press New York, 1960. (Anne Marie Helmenstine has a page linking to a pdf of the book at about.com)
Drink Aware http://www.drinkaware.co.uk/
Notes
Guessing there’s about 300g of glucose in the concentrate, and I know I’ve added 450g sucrose to 5.5 litres of water. As 1 Mole sucrose (242g) yields 2 Moles glucose/fructose (360g), 450g sucrose will make 669g glucose/fructose. With the 300g in the syrup that rounds up to about 1000g total C6H12O6. 1 Mole of C6H12O6 (180g) makes 2 Moles ethanol (92g) plus 2 Moles carbon dioxide (88), so 1000g should make 511g of alcohol and 488g carbon dioxide. That’s roughly half a kilo of alcohol in 5.5kg water, or, ignoring the density difference, about 10% by volume . These kits supposedly deliver 12%, so the 300g estimate was probably low. The volume of gas produced can be calculated given 1 Mole CO2 (44g) has a volume of 22.4 litres at STP (24.6 at current 25deg C room temp), so our 488g equates to 273 litres of gas having to bubble through the airlock. It’s bubbling at about 1 per second with an estimated bubble volume of half a cm3 ; so I figure at that rate it will take 6 days to ferment out. All of which seems to hang together with what it says on the tin.